2024 ASGCT Interview on BiotechTV
2012 Georgia Tech Bio-Industry Symposium Seminar
May 7,2024
TJ Cradick gives us a preview of which abstracts and technologies to watch this year at the ASGCT including the latest in gene editing methods and delivery vehicles. LINK
For Investing In Your Gene Therapy’s Automation Framework
Cell & Gene Virtual Event
December 13, 2023
Considerations For Investing In Your Gene Therapy’s Automation Framework.
TJ Cradick, Ph.D., CSO of Excision BioTherapeutics, and Matt Humes, head of lab automation at Beam Therapeutics, reflect on the biggest advancements they’ve seen throughout their careers. Link to segment. Check out the full digital event available on-demand.
developing a multiplex gene editing approach targeting viral infection
Cell and Gene Therapy Insights
November 8, 2023
David McCall, Senior Editor, Cell & Gene Therapy Insights, speaks to TJ Cradick, Chief Scientific Officer, Excision BioTherapeutics, reflecting on his 20-year career in gene editing and discussing the cutting edge in multiplexing with CRISPR
Examples from the Interview:
Having amassed two decades of experience at the cutting edge of genome editing, what are your high-level reflections on the field today?
Where next for innovation in gene editing platforms? LINK
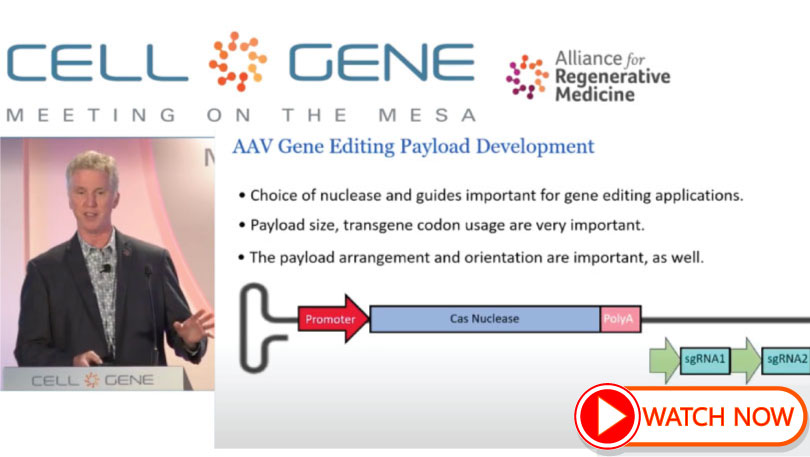
Innovating AAV Vectors to Achieve Better Gene Therapy Outcomes
Meeting on the Mesa Presentation
October 11, 2023
TJ Cradick’s presentation as part of Alliance for Regenerative Medicine (ARM) Meeting on the Mesa session on unique innovations in AAV gene therapies that leverage changes to capsids or the cargo they carry to enable better production, delivery, and therapeutic outcomes. The panelists will also discuss how early, coordinated collaboration across innovation, process development, and CMC teams is essential to reduce risk along the pathway to clinical trials and commercialization.
Panel discussion led by Daniel Teasley with TJ Cradick, Ph.D. Chief Scientific Officer, Excision BioTherapeutics, Rodolphe Clerval, CEO, Coave Therapeutics, An Song, Ph.D., Chief Development Officer, 4DMT.
Excision BioTherapeutics’ CSO Discusses Dual-Guide RNA’s Impact On Gene Editing
Cell and Gene Article
September 15, 2023
By Tyler Menichiello, contributing editor
Excision BioTherapeutics’ gene editing technology uses a novel dual-guide RNA (gRNA) approach to remove large sections of viral DNA in an effort to prevent viral replication and escape. To learn more about this technology and how it fits into the broader scope of gene editing, I met with Excision CSO, Dr. TJ Cradick.
Examples from the Q & A:
Can you explain how Excision’s dual-guide RNA (gRNA) technology came to be?
What makes dual gRNA more effective or better than regular CRISPR Cas-9?
How do you think this technology will impact the field of gene editing?
LINK
TJ Cradick, PhD, on A Potential Cure for HIV With EBT-101
Cell and Gene Therapy Live Interview
October 27, 2022
The chief scientific officer of Excision BioTherapeutics discussed the phase 1/2 clinical trial of the CRISPR gene editing therapy.
CGTLive spoke with TJ Cradick, chief scientific officer, Excision BioTherapeutics, to learn more about the trial and its goals. He discussed the potential of a 1-time infusion to change the HIV treatment landscape. LINK
TJ Cradick, PhD, Presentation and Panel Discussion on World CRISPR Day 2022
World CRISPR Day Presentation and Panel
October 20, 2022
Moving into CRISPR cell and gene therapies, Dr. TJ Cradick was the next speaker in the session. Currently, the CSO of Excision Biotherapeutics, TJ discussed the latest results from a phase I/II trial of the EBT-101 therapy for HIV. By using multiple guide RNAs targeting different regions of the HIV genome, EBT-101 excises the integrated virus from the human genome, offering a one-time cure with minimal chance of viral escape. TJ detailed positive outcomes from preclinical studies of EBT-101 in mice and non-human primates, as well as the current status of human trials. TJ also described other CRISPR therapies currently under development at Excision, including treatments for herpes, JCV, and hepatitis B. LINK
World AIDS Day: A Look Back at the Puzzle
ASGCT Article
December 01, 2022
By Renee Strong
On World AIDS day, Renee Strong, our diversity and inclusion program manager, reflects on her family’s connection with the HIV/AIDS epidemic—and how the work of ASGCT member Dr. TJ Cradick became an essential part of solving the disease puzzle.
Dr. Cradick has been a member of ASGCT since 2005 and is highly regarded in the world of gene editing and HIV/AIDS research.
When Dr. Cradick began his work in Cambridge, Mass., on HIV therapeutics at Repligen, they were sequencing HIV and developing exciting monoclonal antibody approaches that looked promising in primate models. Repligen was also involved in early cell therapy work LINK
EBT-101 achieves robust CRISPR-based editing of HIV proviral DNA without detectable off-target effects.
ASGCT Presentation Movie
May 5, 2022
EBT-101 consists of an adeno-associated virus (AAV) vector expressing the SaCas9 nuclease and 2 gRNAs targeting 3 sites in HIV: 2 well-conserved regions in the 5’ and 3’ long terminal repeats (LTRs) and one in the Gag gene.
Excising these large sections of the integrated HIV-1 genomes disrupts the viral life cycle and is intended to reduce latent HIV-1 patient reservoirs. The efficacy of EBT-101 has been demonstrated in humanized mouse models2
We describe the bioinformatics, as well as our results from multiplex amplicon sequencing that identified high levels of viral excision without detecting unintended indels or recombination.
TJ Cradick Speaks About Their HIV CRISPR Therapy in Trials
CRISPR CUTS
March 31, 2022
In an exciting development for the CRISPR cell and gene therapy field, a CRISPR-based curative therapy for HIV has recently been given FDA approval to begin clinical trials. EBT-101 is the first CRISPR therapy targeting an integrated virus in humans, and it highlights the potential of CRISPR to cure infectious diseases with a single treatment.
To explore this fascinating topic in more detail, we spoke with TJ Cradick, the Chief Scientific Officer of Excision BioTherapeutics, the creators of EBT-101 therapy. We discuss HIV infection and the need for a cure, TJ’s background in HIV research and gene-editing technology, how EBT-101 therapy works, and how Excision Bio is developing CRISPR cures for other viral infections.
Listen to the full interview with TJ Cradick about Excision Bio’s strategy to cure HIV with CRISPR technology. LINK
ASGCT Instructional Video on Gene Editing.
Descriptive cameo by TJ Cradick CAMEO LINK
November 2021
Dr T. J. Cradick explains the difference between in vivo and ex vivo genome editing. The term ‘ex vivo’ means the gene editing components are delivered directly into the body to make edits within the cells, while ex-vivo means the cells are first removed from the patient and then edited in a specialized laboratory and returned back to the patient’s body. Like any medical treatment the potential risks of gene editing are being thoroughly researched.Full Link
TJ Cradick, PhD, ‘Developing EBT-101 CRISPR-Based Therapy for HIV’ as part of World CRISPR Day 2021
World CRISPR Day 2021
October 2021
The final presentation of the Cell & Gene Therapy session was from Dr. TJ Cradick, CSO of Excision BioTherapeutics, who discussed the one-treatment CRISPR-based therapy for HIV known as EBT-101. The talk title was ‘Developing EBT-101 CRISPR-Based Therapy for HIV.’ This strategy uses two guide RNAs to target multiple sites within the HIV genome; by targeting the viral genome and removing it, there is minimal risk of off-target editing. LINK
A CRISPR Vision for the Future of Cell Therapy
CRISPR Journal Online Seminar Series
June 10, 2020
Dr. T.J. Cradick—who was until recently head of genome editing at CRISPR Therapeutics—will introduce how genome editing is used to engineer cell therapies. Several types of cell therapies and their application toward specific disease indications will be demonstrated, including both traditional and current clinical cell therapies. Autogenic, allogeneic, and xenogeneic therapies will be introduced. Autogenic and “off-the-shelf” allogeneic CAR-T cell therapies will be further compared.
Additionally, Dr. Cradick will compare the types of editing that can be used to knock-out or knock-in genes and/or correct sequences. Combinations of these strategies will also be shown in multiplex, advanced editing. Examples will include uses of CRISPR nucleases and more recently developed technologies, base editing, and prime editing. Critical assays and parameters for curative cell therapy will also be discussed. LINK
Design and Validation of Engineered Nucleases for Treating Single-gene Disorders
2012 Georgia Tech Bio-Industry Symposium Seminar
October 2012
As part of the 2012 Georgia Tech Bio-Industry Symposium at the Parker H. Petit Institute for Bioengineering and Bioscience, Georgia Tech faulty member TJ Cradick presented on the ‘Design and Validation of Engineered Nucleases for Treating Single-gene Disorders.’ The presentation covered Zinc Finger Nucleases (ZFNs) and TAL Effector Nucleases (TALENs) and our work on improving TALEN activity and our bioinformatics PROGNOS that scans the genome nominating possible off-target sites. [The presentation slightly preceded our work on CRISPR.] LINK